Abstract
Introduction
Novex® is a biosimilar of Rituximab approved in Argentina having the same indications as the reference product (Mabthera®/Rituxan®). It is commercialized by Laboratorio Elea and since its commercial launch the first national pharmacovigilance plan for a Biosimilar mAb has been implemented.
Material and Methods
In order to detect deviations from expected frequencies of adverse events, a prospective treatment registry for NOVEX® was implemented since beginning of commercialization (November 26th, 2014). The Data Lock Point for this report is June 30th, 2017. Physicians registered the following information for each patient: age, gender, indication, dosing, dose frequency and date of treatment initiation and finalization. Treatment outcome and all Adverse Event detected were registered and coded using MedDRA dictionary.
Results
One-hundred and eighty physicians have participated in this surveillance program. A total of 525 individual records, with at least 1 follow up point, were collected and included in analysis.
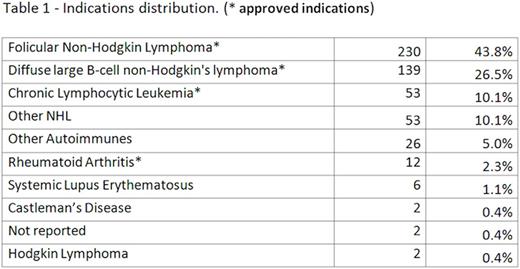
The percentage of female patients was 52%. Age range was 10 - 90 years with a mean age of 63.3 years. The majority of patients have received NOVEX® due to a hematological disease (91.2% of cases) (table 1).
Treatment duration ranged from 154 to 309 days (IQR), with mean of 239 days and median of 214 days. The number of cycles varied from 1 to 12 with a mean of 5.7.
Twenty-four individual Case Safety Reports (ICSRs) were collected, with 29 Adverse Events, and the relative frequency of ICSR was 4.6%. Occurrence rates of adverse events were 1.2 per 100 administered cycles, and 0.022 per 100 treatment days. Fourteen serious adverse events (SAE) were collected and most of them were acute infusion related reactions.
The most frequently reported AEs were:
· Acute Infusion Related Reaction (14)
· Arrhythmia (3)
· Pneumonia (2)
· Stroke (2)
During the program, we detected 41 treatments with rituximab that were started before the launch of the product. Assuming these treatments had begun with Mabthera, this implies the occurrence of interchangeability in this subgroup.
Because of the special interest of this condition, the frequency of ICSRs was analyzed. Two ICSRs were reported, with a relative frequency of 4.9 % (CI95%: 1.7 - 11.5%), which was similar to the whole frequency of the program. Therefore, it was considered that there were no significant differences in the level of reports in the group with interchangeability compared to the general group.
Conclusions
This post-marketing surveillance demonstrates a similar incidence of adverse events after the use of rituximab biosimilar when compared to the published data related to the reference product. Therefore, in terms of tolerability, this biosimilar product has a similar safety profile of that of the reference product. This data is consistent with the high similarity demonstrated during preclinical in vitro and in vivo characterization and the clinical efficacy and safety profile demonstrated in the comparative clinical trial.
Penna: Laboratorio ELEA: Employment. Fernández: Laboratorio ELEA: Employment. Spitzer: Laboratorio ELEA: Employment. Millan: mAbxience: Employment. Gomez: Laboratorio ELEA: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal